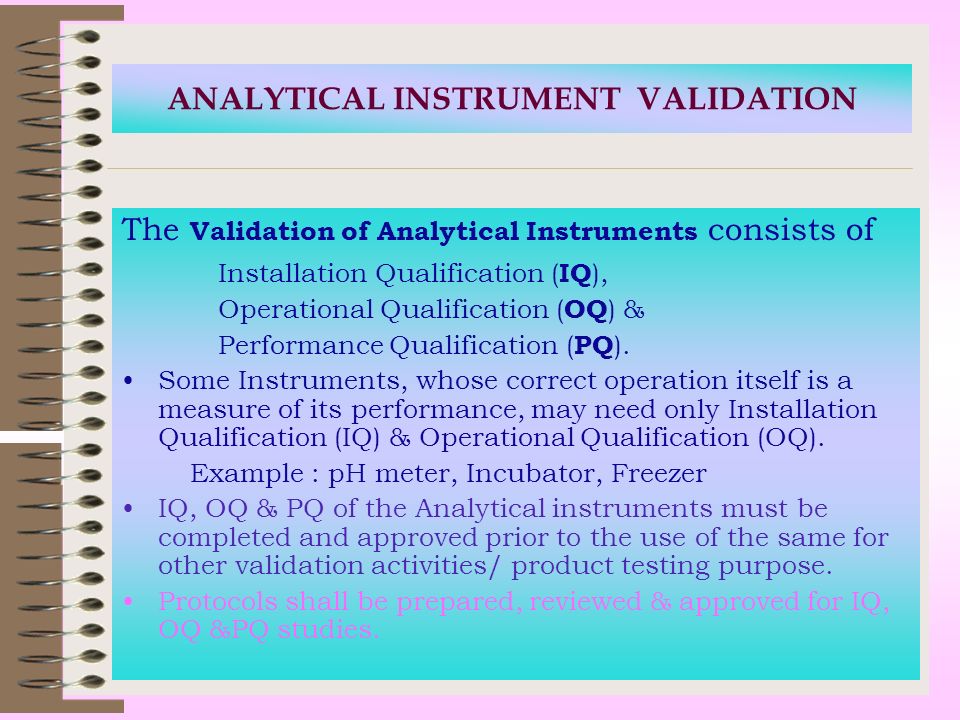
Analytical instrument qualification in capillary electrophoresis - Cianciulli - 2012 - ELECTROPHORESIS - Wiley Online Library
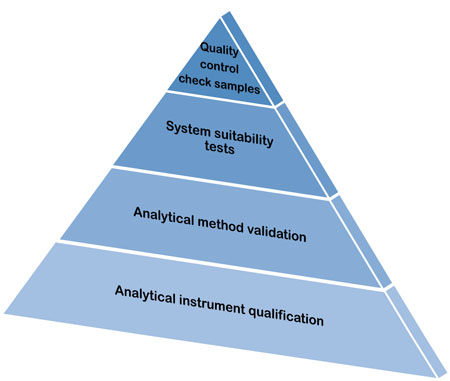
![PDF] AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF PHARMACEUTICAL INDUSTRY | Semantic Scholar PDF] AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF PHARMACEUTICAL INDUSTRY | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/81d9dc060c2784957b63ed435a8b472b79e3af08/2-Figure1-1.png)
PDF] AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF PHARMACEUTICAL INDUSTRY | Semantic Scholar

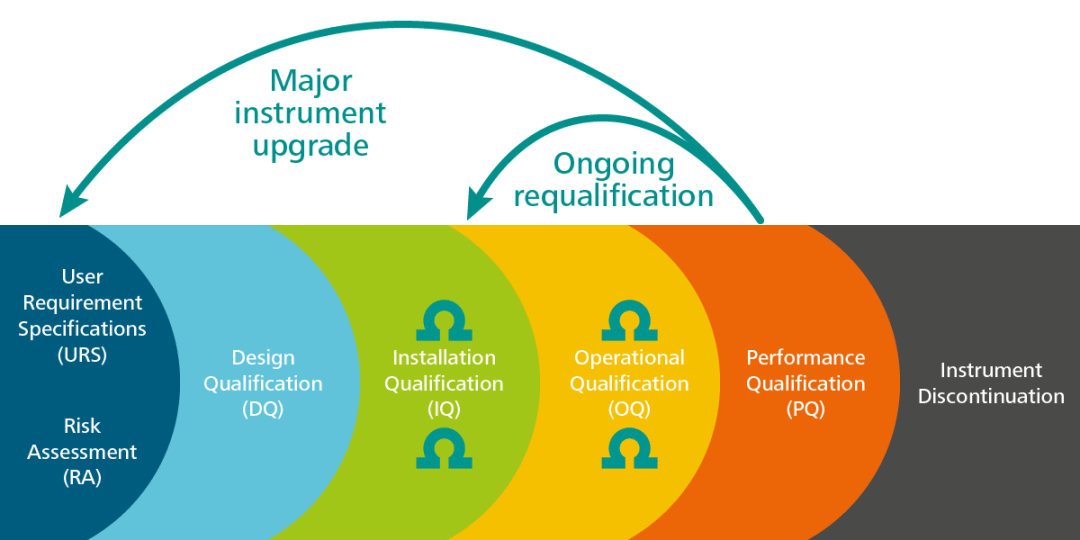
The proper use of certified reference materials for analytical instrumentation qualification | Spectroscopy Europe/World


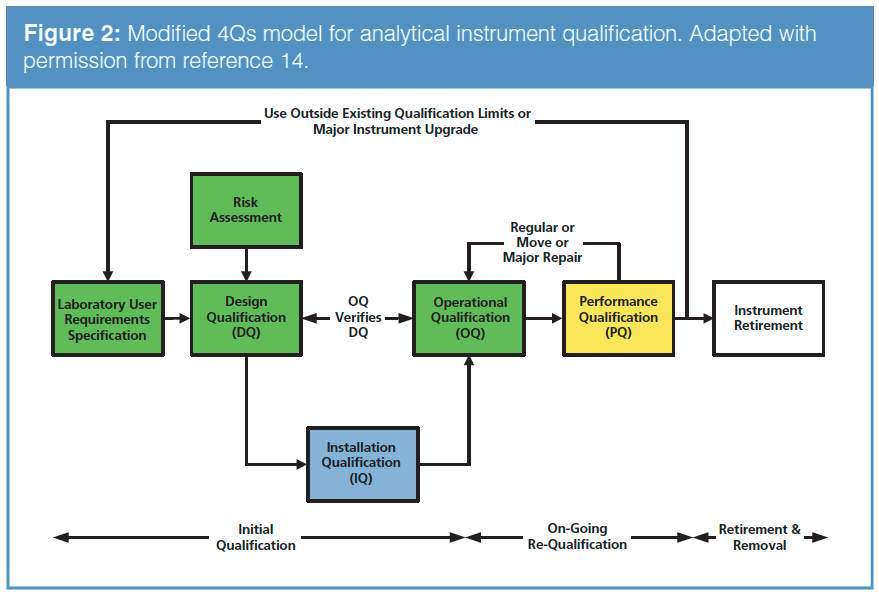
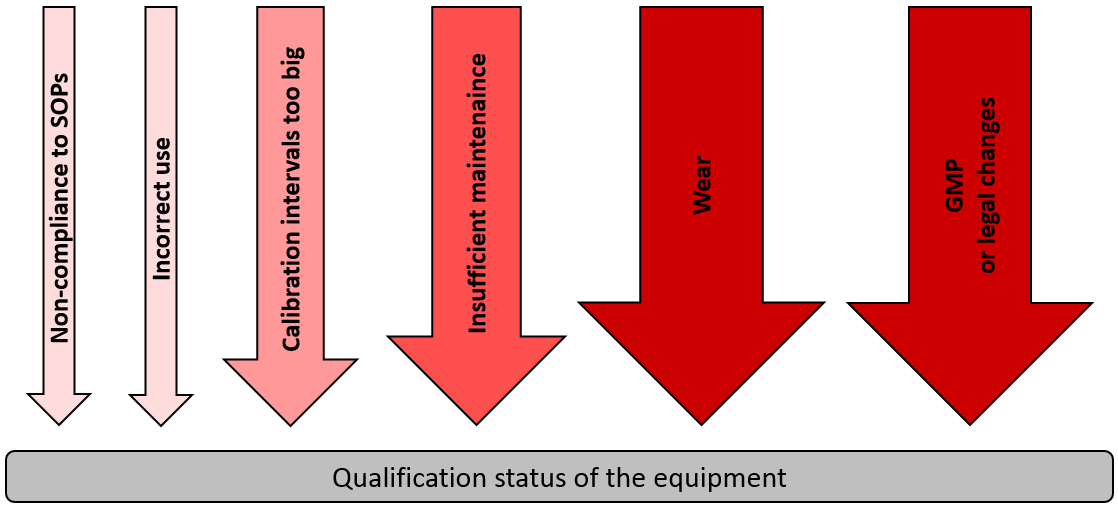
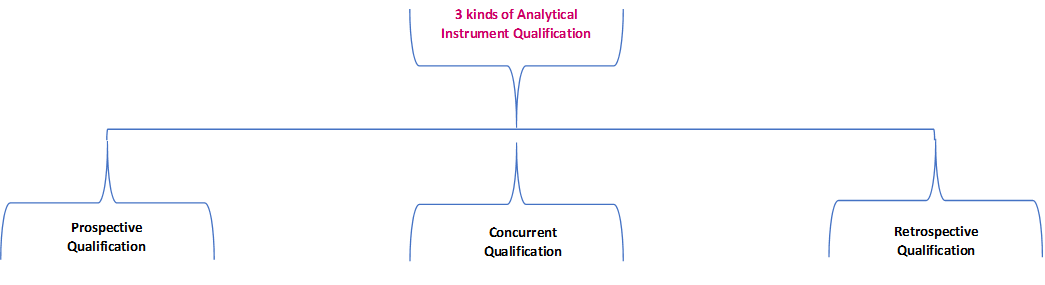
![PDF] á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION | Semantic Scholar PDF] á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/80ff4ccd1aa444bdff6ff42b1127686804f1bd40/2-Figure1-1.png)